Welcome to the Geochemistry Group
The Aqueous Geochemistry and Stable Isotope Mass Spectrometry Group @ SIUC, led by Dr. Liliana Lefticariu, is investigating fundamental and applied aspects of biogeochemical processes occurring at the Earth’s surface. The primary focus of our research is to understand fluid-mediated biogeochemical processes in complex natural and anthropogenic systems using laboratory and field experiments coupled with laboratory analyses and modeling of systems' properties and behavior.
Research Themes
|
Isotope Geochemistry/Analytical Techniques:
Isotope-Ratio Mass Spectrometry (IRMS) is a powerful analytical technique that can accurately measure the relative abundance of isotopes of a chemical element in a given sample. Knowledge of the isotope behavior during physical, chemical, and biological processes is a key to deciphering natural and anthropogenic processes. Isotope ratios can provide exclusive insights into the sources and movement of materials through the geosphere and biosphere and have been applied to problems ranging from climate variability through geological time to deep-crustal processes, and from ecosystem dynamics to diet of ancient humans. Funding for this project was provided to Dr. Lefticariu by U.S. National Science Foundation (NSF-MRI 0821646) and SIUC. The project entitled “Acquisition of an Isotope Ratio Mass Spectrometer for Geochemical Biological and Petrologic Research, Education and Training at Southern Illinois University” has been in place since 2009. [link] |
|
Geochemistry of Natural Waters:
Natural waters provide many benefits to ecosystems and society including agriculture, energy production, industry, and domestic activities. Even though ~70% of the Earth's surface is covered by water, effectively only 3% of the water on the Earth is fresh water, with ~2/3 is stored in glaciers and polar ice caps and ~1/3 is found mainly as groundwater, with a small fraction present as surface freshwater or in the atmosphere. Surface freshwater environments, such as streams, rivers, lakes and freshwater wetlands are important to the above mentioned human activities. The total quantity and quality of freshwater in a given system and at any given time is dependent on both anthropologic and natural factors. Our research has focused on (1) the fundamental understanding of the physical, geochemical, and biological related processes that affect the quality of the fresh-water resources and (2) distinguish between the natural and anthropogenic inputs to the fresh-water systems. This requires knowledge of geology, inorganic and organic chemistry, and microbiology. |
|
Environmental Biogeochemistry:
The environmental impact of human activities includes impacts on freshwater and vegetation, biodiversity and other resources. Acid mine drainage (AMD) is one of the most significant environmental challenges facing mining industry. Although acid drainage is commonly associated with the extraction and processing of sulfide-bearing metalliferous ore deposits and sulfide-rich coal, acidic drainage can occur wherever sulfide minerals are excavated and exposed to atmospheric oxygen. The principal sulfide mineral that can be easily oxidized when exposed to the atmosphere is pyrite, but others are susceptible to oxidation, releasing elements such as aluminum, arsenic, cadmium, cobalt, copper, mercury, nickel, lead, and zinc to the water flowing through the mine waste. The study of intricate processes associated with sulfide oxidation and the formation of acid drainage are of fundamental use in resolving environmental, chemical or geological problems. |
|
Coal Geochemistry:
Coal has been an important energy resource in the US and worldwide. Mining and combustion of coal, however, can create serious environmental problems due to the presence of impurities, such as sulfur, mercury and other trace elements. Deciphering the origin and distribution of the inorganic impurities in coal is critical to the understanding of its technological behavior, by-product potential, geological significance, and environmental impact. |
|
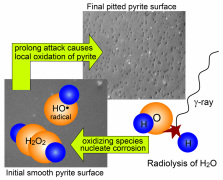
Radiation Geochemistry:
Radiolytic cleavage of water produces a mixture of strong oxidants (e.g., hydrogen peroxide and molecular oxygen) and reductants (e.g., molecular hydrogen), if the products do not quickly recombine as water molecules. The radiolytic processes are particularly significant in geologic environments where molecular oxygen is a negligible input, such as potential biosustainable environments beneath the surface of Mars, including deep vadose zone. Radiolysis is considerably under-recognized as a naturally occurring source of chemical energy for biotic and abiotic reactions. |